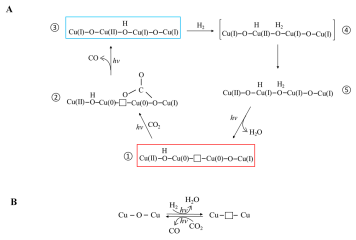
A long-standing challenge in the field of CO2 utilization is how to stabilize Cu2O, an earth-abundant, non-toxic, low-cost (photo)catalyst that can facilitate reduction of CO2 to CO against the irreversible redox disproportionation Cu2O → Cu + CuO, responsible for its instability.
Lili Wan and Wei Sun and coworkers in the Ozin group have solved this problem as reported in their Nature Catalysis paper, by modifying the surface of Cu2O nanocubes with a mixed valence surface frustrated Lewis pair (SFLP), Cu(I,II) ●● OH, which serves to eliminate the redox disproportionation. As depicted in the illustration, H2 undergoes heterolytic dissociation on the SFLP, water is eliminated to create an [O] vacancy and Cu(I) is reduced to Cu(0). Adsorption of CO2 at the [O] vacancy site drives the conversion to CO thereby completing the photocatalytic RWGS reaction cycle
See full article at Nature Catalysis.
-
Recent Posts
- Congratulations to Geoff’s solar ethene and hydrogen paper on Matter
- Congratulations to Geoff’s heterogeneous catalysis paper on Matter
- Congratulations to Geoff’s birthday paper of CO2 photocatalysis on Matter
- Could modified train cars capture carbon from the air? This team has a plan to make it happen
- Sand batteries that are dirt cheap
Recent Comments
Categories
Header Courtesy of Digital Westex