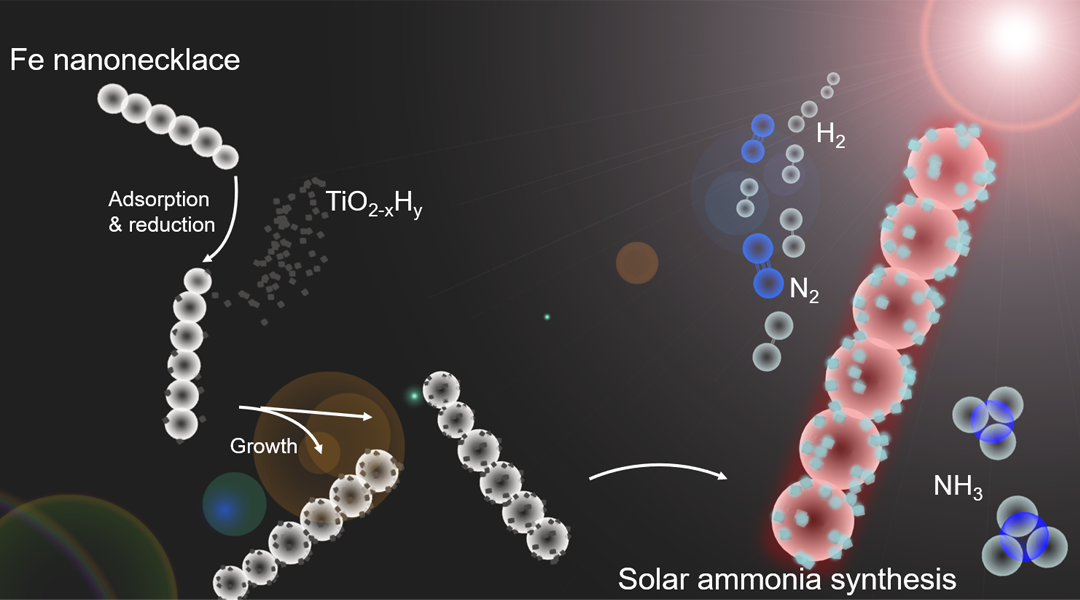
A recent report in which the thermodynamic equilibrium limit of the Haber-Bosch synthesis of ammonia was, for the first time, surmounted by the action of light, and could change the prevailing view of what is possible and not possible in the field of catalysis. A hetero-nanostructured photocatalyst, Fe-TiO2-xHy, which contains both a high and low-temperature site in a single structure has been demonstrated to overcome the thermodynamic equilibrium limit of the ammonia synthesis. This is possible because the exothermic ammonia synthesis process is thermodynamically favored though kinetically sluggish at low temperature; however, the exact opposite prevails at high temperature.
See full article at Advanced Science News.
-
Recent Posts
- Congratulations to Geoff’s solar ethene and hydrogen paper on Matter
- Congratulations to Geoff’s heterogeneous catalysis paper on Matter
- Congratulations to Geoff’s birthday paper of CO2 photocatalysis on Matter
- Could modified train cars capture carbon from the air? This team has a plan to make it happen
- Sand batteries that are dirt cheap
Recent Comments
Categories
Header Courtesy of Digital Westex