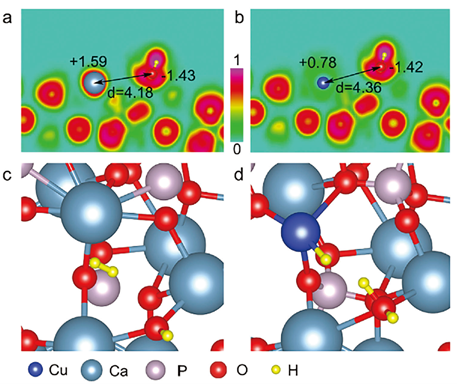
CO2 reduction to commodity chemicals and fuels holds great promise to alleviate the global warming. The realization typically requires earth-abundant catalysts and inexpensive driving force. In this paper, Jiuli and coauthors reported a Cu2+-substituted hydroxyapatite (Ca10(PO4)6(OH)2) mineral photocatalyst achieving peak CO2-to-CO performance of 215 μmol per gram catalyst per hour without CH4 byproduct. The activity originates from novel active sites of surface frustrated Lewis pairs—the proximal Lewis acidic Cu2+ and Lewis basic OH-, which selectively produce CO via a formate intermediate. See full story at Advanced Science.
-
Recent Posts
- Congratulations to Geoff’s solar ethene and hydrogen paper on Matter
- Congratulations to Geoff’s heterogeneous catalysis paper on Matter
- Congratulations to Geoff’s birthday paper of CO2 photocatalysis on Matter
- Could modified train cars capture carbon from the air? This team has a plan to make it happen
- Sand batteries that are dirt cheap
Recent Comments
Categories
Header Courtesy of Digital Westex