Strategies to enhance the carbon capacity of the terrestrial and oceanic sinks tend to focus on landscapes – forest canopy, soil composition, and sea water chemistry. However, we must not neglect the role of animals in maintaining the natural carbon cycle. As it turns out, whales have the capacity to absorb enormous amounts of atmospheric carbon dioxide (CO2). Estimates place the carbon sequestering capacity of a whale to be similar to around 1000 trees with an average whale capturing up to 33 tons of CO2 over its 60-year lifetime, centuries. Efforts to re-establish whale populations, together with large scale reforestation, therefore offer a surprisingly impactful solution to meeting global emission targets.
See full article at Advanced Science News.
-
Recent Posts
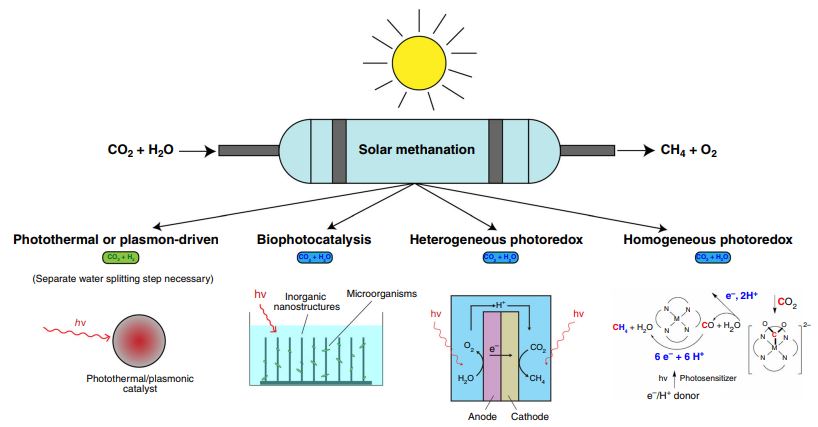
- Congratulations to Geoff’s solar ethene and hydrogen paper on Matter
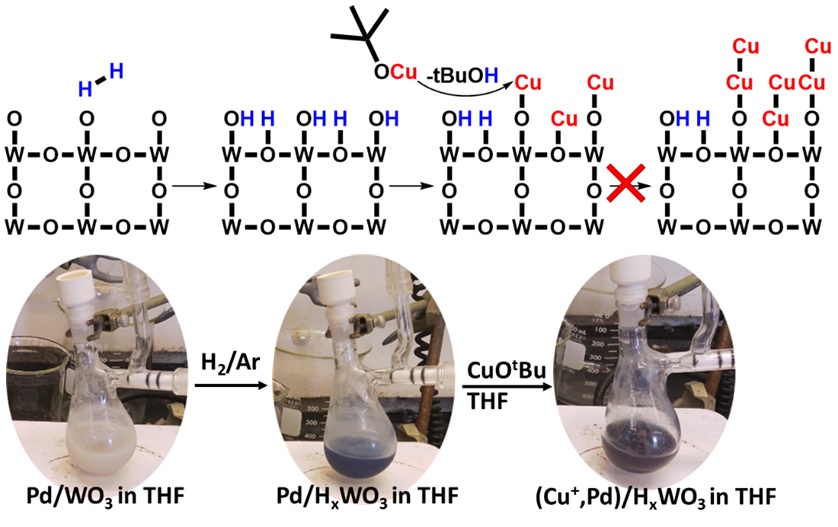
- Congratulations to Geoff’s heterogeneous catalysis paper on Matter
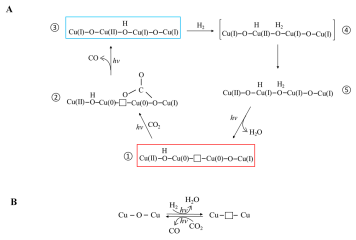
- Congratulations to Geoff’s birthday paper of CO2 photocatalysis on Matter
- Could modified train cars capture carbon from the air? This team has a plan to make it happen
- Sand batteries that are dirt cheap
Recent Comments
Categories
Header Courtesy of Digital Westex