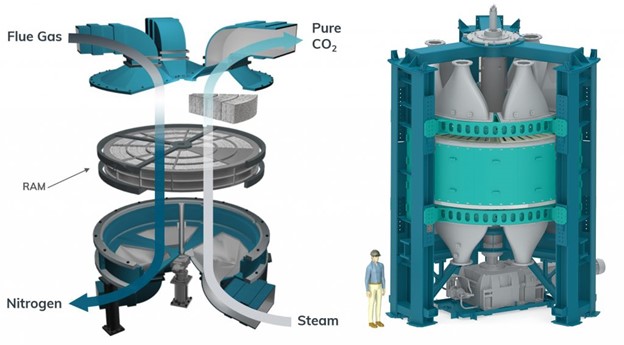
Marketable, large-scale, and energy-efficient CO2 capture is essential for a net-zero future, which would be implemented at high-emitting locations, such as power plants, metal, cement, and chemical refineries. Liquid solvents such as amines, hydroxides, and organics, and metal oxide solids including alkali and alkaline earth oxides are typical absorbents, but they suffer from energy-intensive, non-selective dilemmas. How to develop an efficient material to overcome these problems remains the key challenge. Recently, the CALF-20, an intricate layered crystal composed of zinc, triazolate, and oxalate arranged in a 3D structure filled with nanometer-scale holes, has been used by Canadian company Svante in their revolutionary carbon capture process. A gram of this material has a massive surface area of 500 square meters, and the pores weakly yet preferentially bind CO2 over water. As a result, the CALF-20 displays selective CO2 physisorption at high capacities, retains it up to and beyond 40% RH with durability over 2000 h, and only requires modest amounts of energy to remove CO2. See full story at Advanced Science News.
-
Recent Posts
- Congratulations to Geoff’s solar ethene and hydrogen paper on Matter
- Congratulations to Geoff’s heterogeneous catalysis paper on Matter
- Congratulations to Geoff’s birthday paper of CO2 photocatalysis on Matter
- Could modified train cars capture carbon from the air? This team has a plan to make it happen
- Sand batteries that are dirt cheap
Recent Comments
Categories
Header Courtesy of Digital Westex