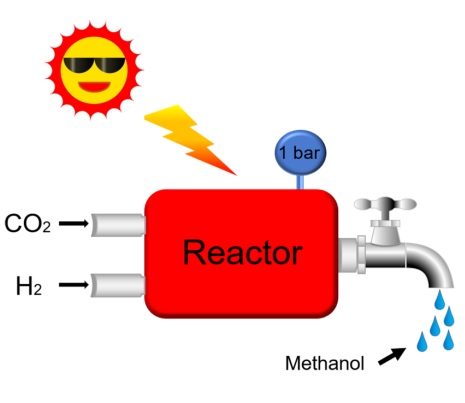
The current fashion for synthesizing methanol continues to be the high pressure and high temperature heterogeneous catalytic conversion of synthesis gas (CO-H2) using alumina supported nanostructured copper-zinc oxide as the catalyst and fossil fuel to power the process. It is an energy intensive process with a large CO2 greenhouse gas footprint and a deleterious effect on the climate. Thus, it would be highly desirable to produce methanol in a sustainable way and use CO2 as feedstock and solar energy to drive the synthesis. Solar technologies that facilitate the efficient conversion of CO2 and H2 into methanol offer a sustainable path to the production of renewable fuels. Furthermore, since about 30% of all known chemicals come from methanol, the production of solar methanol appears to be a “greener” strategy for the chemical and petrochemical industries. In this breakthrough report in Joule, we present a “solar methanol maker”, a rod-shaped In2O3-x(OH)y nanocrystal superstructure, that can efficiently hydrogenate CO2 to methanol at atmospheric pressure with a methanol selectivity for more than 50%. The remarkable production rate of 0.06 mmol gcat-1h-1 and excellent long-term stability of this catalyst in solar methanol synthesis makes it an interesting candidate for converting CO2 to methanol at an industrial scale in a CO2 refinery.
A preview of the study by Chem be read here, along with the full article on the Joule website.